Multiple Choice
Rank the following molecules in order of increasing dipole moment. 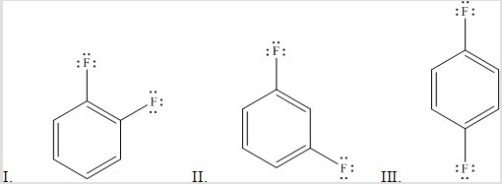
A) III < II < I
B) III < I < II
C) I < II < III
D) I < III < II
E) II < III < I
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q129: Which has a dipole moment of zero?<br>A)
Q130: What is the molecular geometry of BeH<sub>2</sub>
Q131: Which is the most reasonable prediction for
Q132: Indicate the type of hybrid orbitals used
Q133: What is the hybridization of the As
Q134: How many π bonds are there in
Q135: What is the molecular geometry of ClF<sub>2</sub><sup>-</sup>
Q136: Using the VSEPR model, predict the molecular
Q138: According to the VSEPR model, the predicted
Q139: In which molecule is the central atom