Multiple Choice
Allene, C3H4 (shown below) , is one component of gas used for high-temperature welding. According to the valence bond model, which is a proper description of the bonding on the central carbon atom in allene? 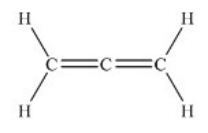
A) It shares four 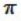 bonds, two with each carbon atom on either side.
bonds, two with each carbon atom on either side.
B) It shares four σ bonds, two with each carbon atom on either side.
C) It shares one σ bond and one 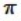 bond with each carbon atom on either side.
bond with each carbon atom on either side.
D) It shares two σ bonds with the carbon atom on the left, and two 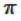 bonds with the carbon atom on the right.
bonds with the carbon atom on the right.
E) It shares two σ bonds with the carbon atom on the right, and two 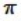 bonds with the carbon atom on the left.
bonds with the carbon atom on the left.
Correct Answer:

Verified
Correct Answer:
Verified
Q25: Indicate the type of hybrid orbitals used
Q26: Which molecular formula corresponds to a structural
Q27: What is the total number of electron
Q28: How many electron domains are on the
Q29: The hybridization of the central nitrogen atom
Q31: The PCl<sub>5</sub> molecule has<br>A) nonpolar bonds, and
Q32: The number of π bonds in phosgene,
Q33: What is the molecular geometry of HOF
Q34: What is the number of lone electron
Q38: The BrF<sub>5</sub> molecule has polar bonds and