Multiple Choice
Which formula is incorrectly matched with its VSEPR model representation? (Note: Lone pairs on the models, if any, are not shown.)
A) 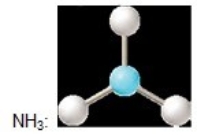
B) 
C) 
D) 
E) 
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q120: What is the hybridization on the central
Q121: Which of the following is required for
Q122: What is the total number of electron
Q123: Which of the following molecules has a
Q124: Valence bond theory predicts that iodine will
Q126: Indicate the type of hybrid orbitals used
Q127: When PCl<sub>5</sub> solidifies it forms PCl<sub>4</sub><sup>+</sup>cations and
Q128: The bond angle for an sp hybrid
Q129: Which has a dipole moment of zero?<br>A)
Q130: What is the molecular geometry of BeH<sub>2</sub>