Multiple Choice
Thiocarbonyl disulfide (CSF2) , based on the coordinate axes provided below, in which direction does the net molecular dipole moment point for the molecule? 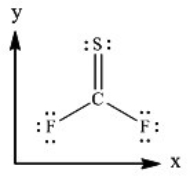
A) It points in the +x direction.
B) It points in the -x direction.
C) It points in the +y direction.
D) It points in the -y direction.
E) The molecule does not have a net molecular dipole moment.
Correct Answer:

Verified
Correct Answer:
Verified
Q1: What is the total number of electron
Q2: How many bonds are there in one
Q3: Which one of the following molecules is
Q5: According to the VSEPR model, is the
Q6: According to molecular orbital (MO) theory, the
Q7: Predict the molecular geometry and polarity of
Q8: How do you describe the molecular geometry
Q9: What is the molecular geometry of N<sub>2</sub>O
Q10: Carbon uses _ hybrid orbitals in ClCN.<br>A)
Q11: In the valence bond treatment, a π