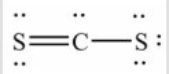Related Questions
Q52: Describe in brief how electronegativity values can
Q54: Select the compound with the highest (i.e.,
Q90: Which of these compounds is most likely
Q91: What types of elements undergo ionic bonding?<br>A)
Q92: Which of these compounds is most likely
Q94: Which of these ionic solids would have
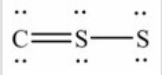
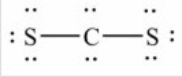
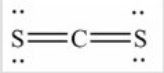
Q97: How many lone pairs of electrons need
Q98: What is the electrostatic attraction called that
Q99: The formal charge on Cl in the
Q100: A(n) _ is a representation of covalent