Multiple Choice
In the following Lewis structure for ClO3F, chlorine has a formal charge of ________ and an oxidation number of ________. 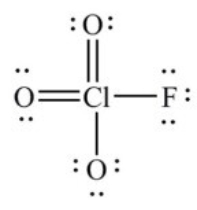
A) 7, 7
B) 7, -1
C) 1, 1
D) 1, -1
E) 1, 7
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q8: Which of the following contains ionic bonding?<br>A)CO<br>B)SrF
Q20: Which of the following is an ionic
Q33: What is the formal charge on phosphorus
Q51: The Lewis structure for a chlorate ion,
Q52: What name is given to the sharing
Q53: How many total resonance structures can be
Q55: The Lewis dot symbol for the a
Q59: Which of these compounds is most likely
Q61: Which statement is false concerning ionic bonds
Q129: In the Lewis structure of the iodate