Multiple Choice
What is ΔH°rxn for the decomposition of calcium carbonate to calcium oxide and carbon dioxide? CaCO3(s) → CaO(s) + CO2(g) 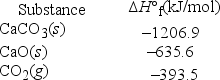
A) -2236.0 kJ/mol
B) -1449.0 kJ/mol
C) -177.8 kJ/mol
D) 177.8 kJ/mol
E) 2236.0 kJ/mol
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q71: Which of the following processes always results
Q76: Your favorite candy bar, Gummy Beakers, contains
Q77: What is the standard enthalpy of formation
Q78: What law states that energy can be
Q79: A 1.00-g sample of octane (C<sub>8</sub>H<sub>18</sub>) is
Q80: Which is not a state function?<br>A) internal
Q83: Ethanol, C<sub>2</sub>H<sub>5</sub>OH, is promoted as a clean
Q84: For a particular process, 28 kJ of
Q85: Consider the following two representations of chemical
Q86: If 325 g of water at 4.2°C