Multiple Choice
Using Hess' law, what is ΔH°rxn at 25°C for the following reaction? ClF(g) + F2(g) → ClF3(g) 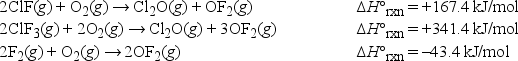
A) -217.5 kJ/mol
B) -130.2 kJ/mol
C) 217.5 kJ/mol
D) -108.7 kJ/mol
E) 465.4 kJ/mol
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q21: In an endothermic reaction, in going from
Q96: When 0.560 g of Na(s) reacts with
Q97: To which one of the following reactions,
Q98: What is ΔH°<sub>rxn</sub> for the following reaction?
Q99: How much heat is released if 35.0
Q100: Calculate the standard enthalpy change for the
Q102: The heat absorbed by a system at
Q103: Which equation has a ΔH<sub>rxn</sub> that is
Q104: How much heat is required to raise
Q105: In which reaction would you expect ΔH