Multiple Choice
Suppose a 0.500-g sample of an organic compound is analyzed via bomb calorimetry. The temperature of the calorimeter is measured over time. At t = 5 min, the combustion reaction is initiated. Below is a plot of the data that are obtained. 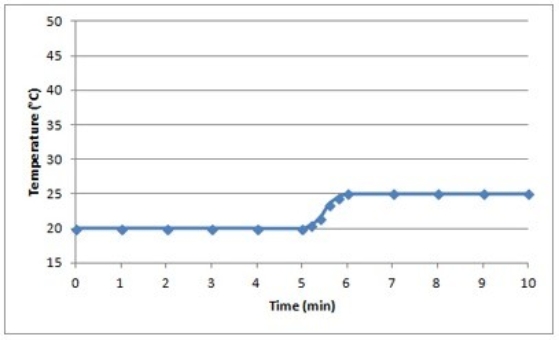 Suppose the experiment is repeated under identical conditions, but with a 1.000-g sample of the organic compound. What might a plot of the resulting data look like?
Suppose the experiment is repeated under identical conditions, but with a 1.000-g sample of the organic compound. What might a plot of the resulting data look like?
A) 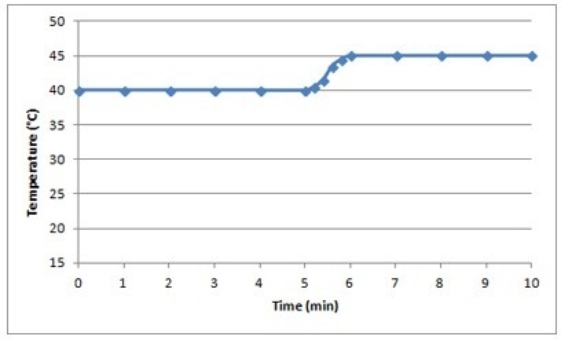
B) 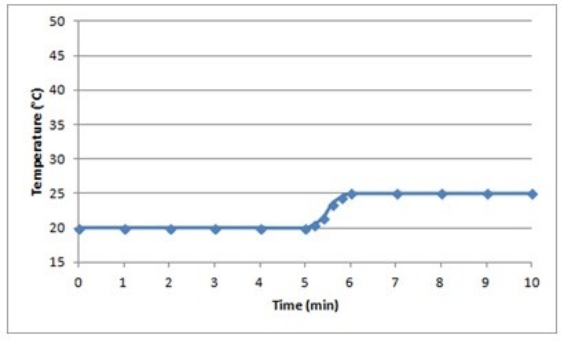
C) 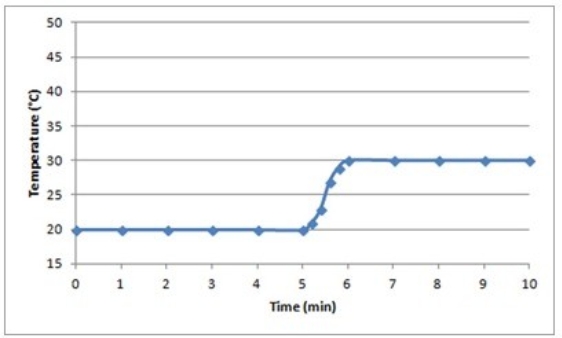
D) 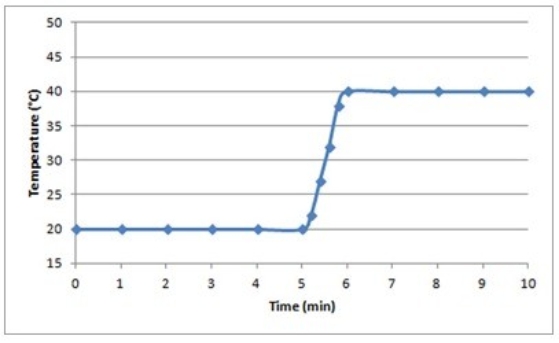
E) 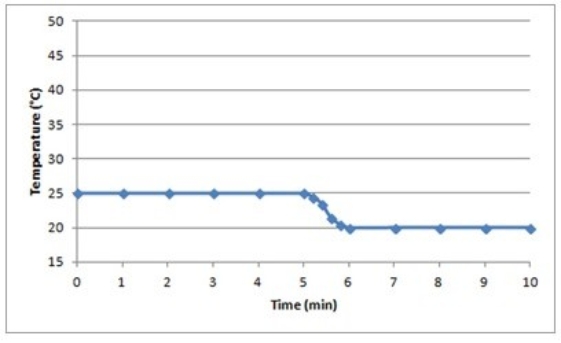
Correct Answer:

Verified
Correct Answer:
Verified
Q28: A(n) _ _ is a process where
Q29: What is q if 28.6 g of
Q30: Based on the following thermochemical equations, what
Q31: Thunderstorms are powered by the heat released
Q32: The enthalpy change for making one mole
Q34: A system delivers 1275 J of heat
Q35: Which of the following is incorrectly matched?<br>A)
Q36: What is the change in temperature if
Q49: At body temperature 2,404 joules of energy
Q100: A home aquarium is an example of