Multiple Choice
Which balanced chemical equation could represent the following result of a precipitation reaction? 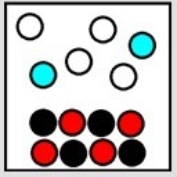
A) 2K3PO4(aq) + 3Cr2(SO4) 3(aq) → CrPO4(s) + 6KCl(aq)
B) MgSO4(aq) + 2AgNO3(aq) → Ag2SO4(s) + Mg(NO3) 2(aq)
C) MgCl2(aq) + 2AgC2H3O2(aq) → 2AgCl(s) + Mg(C2H3O2) 2(aq)
D) K2CrO4(aq) + 2AgNO3(aq) → Ag2CrO4(s) + 2KNO3(aq)
E) K2S(aq) + Pb(NO3) 2(aq) → PbS(s) + 2KNO3(aq)
Correct Answer:

Verified
Correct Answer:
Verified
Q57: Which salt is produced by the neutralization
Q58: If aqueous solutions of Mg(C<sub>2</sub>H<sub>3</sub>O<sub>2</sub>)<sub>2</sub> and LiOH
Q59: Which is a strong base?<br>A) LiOH<br>B) CH<sub>3</sub>COOH<br>C)
Q60: A 50.0 mL sample of 0.436 M
Q62: Which salt is formed in the neutralization
Q63: OH<sup>-</sup> is called the _ _.
Q65: Which of these chemical equations describes a
Q66: Given the following information, write a short
Q115: A 250. mL sample of 0.0328M HCl
Q133: Which of these compounds is a nonelectrolyte?<br>A)