Multiple Choice
Which represents the result of mixing equal volumes of 1 M HCl and 1 M NH3 solutions? (Each sphere represents 1 mol of ions.)
A) 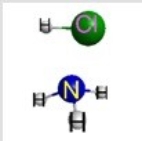
B) 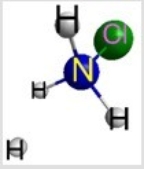
C) 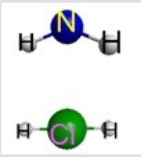
D) 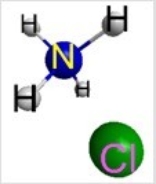
E) 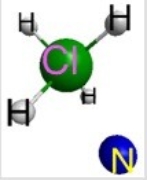
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q37: The distinguishing characteristic of all nonelectrolyte solutions
Q50: Which of the following is a strong
Q76: Which substance is present in the smallest
Q100: Automobile batteries use 3.0 M H<sub>2</sub>SO<sub>4</sub> as
Q108: Based on the solubility rules, which one
Q109: A 3.682-g sample of KClO<sub>3</sub> is dissolved
Q110: Based on the solubility rules, which of
Q114: Based on the solubility rules, which one
Q118: Which is a strong acid?<br>A) Ba(OH)<sub>2</sub><br>B) H<sub>3</sub>PO<sub>4</sub><br>C)
Q122: Which substance is present in the largest