Multiple Choice
Which is a representation of a balanced chemical equation for the reaction of nitrogen gas and chlorine gas to form nitrogen trichloride?
A) 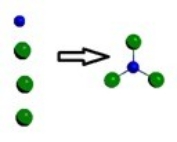
B) 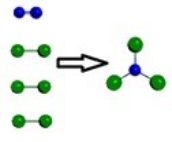
C) 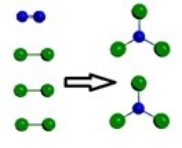
D) 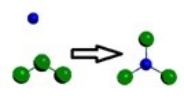
E) 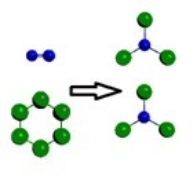
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q7: What is the mass in grams of
Q42: Sulfur dioxide reacts with chlorine to produce
Q44: Calculate the number of moles in 17.8
Q80: The percent composition by mass of a
Q100: What mass of nitrogen gas is required
Q102: The percent yield can be determined by
Q104: The limiting reactant is the reactant with
Q106: What is the percent carbon in CH<sub>3</sub>CH<sub>2</sub>OH?<br>A)
Q107: What is the theoretical yield of calcium
Q108: What is the coefficient of H<sub>2</sub>O when