Multiple Choice
Which one of the atoms shown would be most likely to form a cation with a charge of +1?
A) 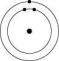
B) 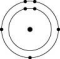
C) 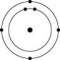
D) 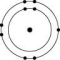
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q9: Which of the following are compounds?<br>A) H₂O,
Q15: Compared with ³¹P, the radioactive isotope ³²P
Q16: Trace elements are those required by an
Q22: A salamander relies on hydrogen bonding to
Q26: How many electron pairs are shared between
Q43: We can represent atoms by listing the
Q44: If an atom has a charge of
Q48: Refer to the following figure to answer
Q50: You are asked to indicate the type
Q61: A covalent bond is likely to be