Multiple Choice
Refer to the following figure to answer the questions below.
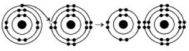
-What causes the shape of the molecule shown?
A) the shape of the two p orbitals in the carbon atom
B) the shape of the one s orbital in the carbon atom
C) the shape of the sp³ hybrid orbitals of the electrons shared between the carbon and hydrogen atoms
D) hydrogen bonding configurations between the carbon and hydrogen atoms
Correct Answer:

Verified
Correct Answer:
Verified
Q18: Carbon-14 has the same _.<br>A) atomic number
Q20: What coefficients must be placed in the
Q22: Refer to the following figure to answer
Q24: Which of the following is broken when
Q26: How many electron pairs are shared between
Q27: Which of the following is the best
Q28: A neutral atom has two, eight, eight
Q40: Oxygen has an atomic number of 8
Q45: In the term trace element, the adjective
Q49: Nitrogen (N) normally forms three covalent bonds