Multiple Choice
The solutions in the arms of a U-tube are separated at the bottom of the tube by a selectively permeable membrane. The membrane is permeable to sodium chloride but not to glucose. Side A is filled with a solution of 0.4 M glucose and 0.5 M sodium chloride (NaCl) , and side B is filled with a solution containing 0.8 M glucose and 0.4 M sodium chloride. Initially, the volume in both arms is the same.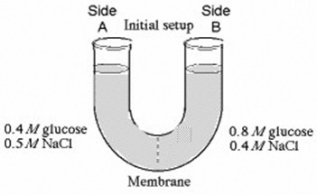
In the U-tube experiment illustrated above, which of the following statements correctly describes side B at equilibrium?
A) The concentration of NaCl and glucose will decrease, and the water level will increase.
B) The concentration of NaCl will increase, the concentration of glucose will decrease, and the water level will increase.
C) The concentration of NaCl will increase, the concentration of glucose will decrease, and the water level will decrease.
D) The concentration of NaCl and glucose will increase, and the water level will decrease.
Correct Answer:

Verified
Correct Answer:
Verified
Q13: For a protein to be an integral
Q23: A bacterium engulfed by a white blood
Q27: Diffusion of ions across membranes through specific
Q39: The membranes of winter wheat are able
Q44: The force driving simple diffusion is _,
Q52: The solutions in the two arms of
Q56: The solutions in the two arms of
Q57: For the following questions, match the labeled
Q64: Which of the following structures would decrease
Q64: A phospholipid bilayer with equal amounts of