Multiple Choice
According to the Ideal Gas Law, 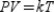 , where P is pressure, V is volume, T is temperature (in Kelvins) , and k is a constant of proportionality. A tank contains 2,500 cubic inches of nitrogen at a pressure of 30 pounds per square inch and a temperature of 700 K. Determine k.
, where P is pressure, V is volume, T is temperature (in Kelvins) , and k is a constant of proportionality. A tank contains 2,500 cubic inches of nitrogen at a pressure of 30 pounds per square inch and a temperature of 700 K. Determine k.
A) 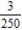
B) 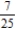
C) 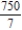
D) 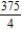
E) 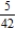
Correct Answer:

Verified
Correct Answer:
Verified
Q29: Find the limit. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8527/.jpg" alt="Find
Q30: Examine the function <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8527/.jpg" alt="Examine the
Q31: Suppose a home improvement contractor is painting
Q32: Find and simplify the function <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8527/.jpg"
Q33: Suppose a company manufactures two types of
Q35: Find a unit normal vector to the
Q36: Find the maximum value of the directional
Q37: Find the minimum distance from the point
Q38: Find the limit . <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8527/.jpg"
Q39: Use polar coordinates to find the limit