Short Answer
Neon has three isotopes as shown in the table.
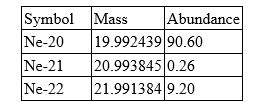 Calculate the atomic mass of neon. Include all digits in your answer.
Calculate the atomic mass of neon. Include all digits in your answer.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q9: The full electronic configuration for Br is
Q10: Calculate the energy emitted from a potassium
Q11: The abbreviated electron configuration for xenon is
Q12: Which two Greek philosophers had conflicting thoughts
Q13: Which of the following statements is NOT
Q14: Explain how a line spectrum is made.
Q15: The full electronic configuration for Ca is
Q16: Which of the following configurations is the
Q18: Explain the difference between the law of
Q19: Which scientist was responsible for the discovery