Multiple Choice
Which of the following is the correct electronic configuration for an Mn atom with 25 electrons?
A) 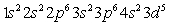
B) 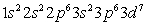
C) 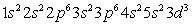
D) 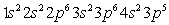
E) 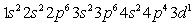
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: The first two columns of the periodic
Q2: Electrons in the last unfilled subshell of
Q3: Which of the following is the partially
Q4: Which of the following magnetic quantum numbers
Q5: The wavelength of light is 3 ×
Q7: Atomic radii decrease as you go down
Q8: Electrons within a shell will have the
Q9: A _ spectrum is an image that
Q10: The _ principle states that no two
Q11: The valence shell electron configuration of the