Multiple Choice
Which of the following equations represent the ideal gas law?
A) P1V1 = P2V2
B) 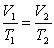
C) 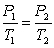
D) 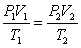
E) PV = nRT
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q66: A 3.8 mL volume of gas contains
Q67: A sample of gas has a volume
Q68: A mixture of helium at 1.600 atm
Q69: A sample of a gas has an
Q70: The _ of a gas is the
Q72: A gas has an initial pressure of
Q73: _ law states that P × V
Q74: Pressure and volume of a gas are
Q75: How many moles of H<sub>2</sub> are present
Q76: A sample of gas has an initial