Multiple Choice
Use the following information to answer the question below. 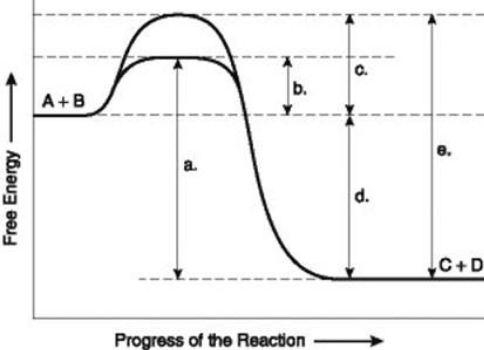 The figure illustrates the energy states associated with the reaction A + B ↔ C + D. Which of the following terms best describe the forward reaction in the figure?
The figure illustrates the energy states associated with the reaction A + B ↔ C + D. Which of the following terms best describe the forward reaction in the figure?
A) endergonic, ∆G > 0
B) exergonic, ∆G < 0
C) endergonic, ∆G < 0
D) exergonic, ∆G > 0
Correct Answer:

Verified
Correct Answer:
Verified
Q17: Which of the following statements is the
Q18: Which of the following statements best describes
Q19: A decrease in entropy is associated with
Q20: In the citric acid cycle, succinate dehydrogenase
Q21: The ΔG of ATP hydrolysis in a
Q23: Which of the following statements best describes
Q24: Use the following information to answer the
Q25: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8578/.jpg" alt=" Which of the
Q26: Choose the pair of terms that correctly
Q27: In the citric acid cycle, succinate dehydrogenase