Multiple Choice
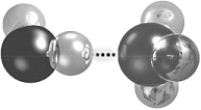
-The dots in the figure represent a(n)
A) covalent bond.
B) ionic bond.
C) hydrogen bond.
D) polar covalent bond.
E) hydrophobic interaction.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q30: Isotopes of atoms<br>A) are electrically unbalanced.<br>B) behave
Q31: The atomic mass (mass number) of an
Q32: A solution with a pH of 9
Q33: Nitrogen, with an atomic number of 7,
Q34: Which is NOT an element?<br>A) water<br>B) oxygen<br>C)
Q36: Classification.The following are types of chemical bonds.Answer
Q37: "Acidic" is an appropriate description for all
Q38: Which statement concerning radioisotope <sup>14</sup>C is false?<br>A)
Q39: Sodium chloride (KCl) in water can be
Q40: The column of water extending in tubes