Multiple Choice
The boiling point of water at sea level is 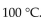
At higher altitudes, the boiling point of water will be
A) higher, because the altitude is greater.
B) the same, because water always boils at 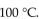
C) lower, because the atmospheric pressure is lower.
D) higher, because there are fewer water molecules in the air.
E) lower, because temperatures are lower.
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Vapor pressure can be described as<br>A)the pressure
Q2: The pressure of 5.0 L of gas
Q3: The volume occupied by a gas is
Q4: The pressure exerted by the particles of
Q6: A barometer is a device for measuring
Q7: Which of the following correctly describes the
Q8: The volume of a sample of gas,
Q9: Which of the following is NOT part
Q10: What volume would a 0.250
Q11: The mathematical expression of the ideal gas