Multiple Choice
Avogadro's number is the number of
A) grams in 1 mole of a substance.
B) moles in 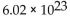
grams of an element.
C) moles in 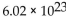
amu of an element.
D) amu in 1 mole of a substance.
E) particles in 1 mole of a substance.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q41: 0.100 mole of lithium has a mass
Q42: What is the molar mass of copper(II)
Q43: The _ is the energy difference between
Q44: One mole of neon has a mass
Q45: What is the mass of 0.425 mole
Q47: How many kcal are produced when
Q47: For the following question(s),consider the following equation.<br>2Mg
Q48: Calculate the molar mass of magnesium chloride,
Q50: For the question(s)that follow, consider the following
Q51: In this reaction, what is the substance