Multiple Choice
When 10.0 g of 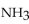 reacts, the actual yield of
reacts, the actual yield of 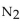 is 8.50 g. What is the percent yield?
is 8.50 g. What is the percent yield?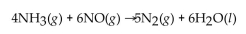
A) 20.6 %
B) 8.5 %
C) 41.3 %
D) 85.0 %
E) 51.5 %
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q17: One mole of helium gas has a
Q18: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8494/.jpg" alt=" A)206
Q20: Which of the following is an oxidation-reduction
Q21: What type of reaction is the following?
Q23: The reaction of carbon with oxygen to
Q24: Which of the following gives the balanced
Q25: One mole of particles of any substance
Q26: In the reaction of nitrogen gas, <img
Q27: The number of molecules in 1 mole
Q45: Pentane ( C<sub>5</sub>H<sub>12</sub> )reacts with oxygen