Multiple Choice
Barium chloride and sodium sulfate react according to the following equation. 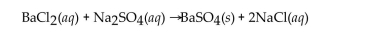 Answer the question(s) that follow about this reaction.
Answer the question(s) that follow about this reaction.
-How many grams of barium chloride are needed to make 100. grams of barium sulfate?
A) 46.6 g
B) 233.3 g
C) 44.9 g
D) 89.2 g
E) 208.3 g
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q71: What is the molar mass of helium?
Q72: What is oxidized and what is reduced,
Q73: How many moles of water, H<sub>2</sub>O,
Q74: How many moles of magnesium are needed
Q75: When 3.05 moles of <img
Q77: In the reaction of silver nitrate with
Q78: What type of reaction is the following?
Q79: How many grams of hydrogen are needed
Q80: In any balanced chemical equation, the number
Q81: What is the molar mass of sodium