Multiple Choice
The correct formula for a compound formed from the elements Al and O is
A) 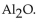
B) 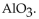
C) 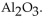
D) AlO.
E) 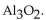
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q26: How many electrons will aluminum gain or
Q27: Which one of the following compounds contains
Q28: How many valence electrons are in the
Q29: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8494/.jpg" alt=" A)nitrogen trioxide. B)dinitride
Q30: The ion of aluminum is<br>A) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/.jpg"
Q32: Elements in group 2A (2)of the periodic
Q33: How many valence electrons are in the
Q34: The number of electrons in an ion
Q35: The ability of an atom to attract
Q36: How many electrons will iodine gain or