Multiple Choice
How many lone pairs of electrons are in the Lewis structure of 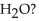
A) 0
B) 1
C) 2
D) 3
E) 4
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q65: An ionic compound<br>A)has a net negative charge.<br>B)has
Q66: To form an ion, a sodium atom<br>A)gains
Q67: The name of the HSO4- ion is<br>A)sulfate.<br>B)hydrogen
Q68: The shape of the carbon tetrachloride molecule
Q69: The carbon tetrachloride molecule, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/.jpg" alt="The
Q71: The name of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/.jpg" alt="The name
Q72: How many electrons will chlorine gain or
Q73: Which of the following compounds contains a
Q74: The correct name of the compound <img
Q75: The correct name for the compound <img