Multiple Choice
The ammonia molecule, 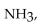 is
is
A) a nonpolar molecule with nonpolar bonds.
B) a polar molecule with ionic bonds.
C) a polar molecule with polar bonds.
D) a nonpolar molecule with polar bonds.
E) a polar molecule with nonpolar bonds.
Correct Answer:

Verified
Correct Answer:
Verified
Q41: The number of valence electrons found in
Q42: The strongest interactions between molecules of iodine,
Q43: The name of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/.jpg" alt="The name
Q44: The strongest interactions between atoms of helium,
Q45: The compound <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/.jpg" alt="The compound
Q47: Which of the following polyatomic ions has
Q48: The strongest interactions in the compound sodium
Q49: The carbon tetrachloride molecule, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/.jpg" alt="The
Q50: In a covalently bonded molecule, the number
Q51: What is the correct formula for iron(III)