Multiple Choice
Which of the following electron configurations is impossible?
A) 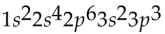
B) 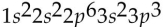
C) 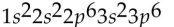
D) 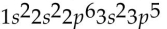
E) 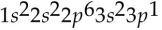
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q5: Consider a neutral atom with 30 protons
Q67: What is the symbol of the element
Q68: Ca is the symbol for<br>A)cadmium.<br>B)copper.<br>C)cobalt.<br>D)calcium.<br>E)carbon.
Q69: Which of the following properties is NOT
Q70: Ionization energy is<br>A)the energy an ion acquires
Q71: What is the abbreviated electron configuration for
Q74: Isotopes are atoms of the same element
Q75: The number of neutrons in an atom
Q76: Which of the following elements is a
Q77: The number of electron levels in a