Multiple Choice
In a sulfuric acid solution, where the 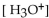 is 0.01 M, what is the pH?
is 0.01 M, what is the pH?
A) pH=11.0
B) pH=12.0
C) pH=5.0
D) pH=2.0
E) pH=3.0
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q10: What is the <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/.jpg" alt="What is
Q11: The conjugate acid of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/.jpg" alt="The
Q12: The conjugate base of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/.jpg" alt="The
Q13: The name of HCl is_.
Q14: In which of the following are the
Q16: 25.0 mL of 0.212 M NaOH is
Q17: When hyperventilation (rapid breathing)causes a patient to
Q18: Which one of the following is characteristic
Q19: When a piece of magnesium metal is
Q20: An acid and base react to form