Multiple Choice
A 25.0 mL sample of 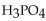 requires 50.0 mL of 1.50 M NaOH for complete neutralization. What is the molarity of the acid?
requires 50.0 mL of 1.50 M NaOH for complete neutralization. What is the molarity of the acid?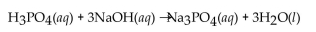
A) 0.750 M
B) 0.333 M
C) 1.50 M
D) 1.00 M
E) 3.00 M
Correct Answer:

Verified
Correct Answer:
Verified
Q37: Identify the conjugate acid-base pairs in the
Q38: The function of a buffer is to<br>A)maintain
Q39: The name given to an aqueous solution
Q40: Which of the following is the strongest
Q41: The normal blood pH is about<br>A)7.00.<br>B)7.20.<br>C)7.40.<br>D)6.80.<br>E)7.60.
Q43: Which solution has the highest pH?<br>A)a buffer
Q44: Which of the following is the weakest
Q45: The name of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/.jpg" alt="The name
Q46: How many moles of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/.jpg" alt="How
Q47: How many milliliters of 0.400 M NaOH