Multiple Choice
A 10.0 mL of 0.121 M 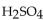 is neutralized by 17.1 mL of KOH solution. The molarity of the KOH solution is
is neutralized by 17.1 mL of KOH solution. The molarity of the KOH solution is
A) 0.142 M
B) 0.428 M
C) 0.207 M
D) 0.0708 M
E) 0.4141 M
Correct Answer:

Verified
Correct Answer:
Verified
Q27: Write the proper acid dissociation expression for
Q28: A solution which has <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/.jpg" alt="A
Q29: In a neutralization reaction,<br>A)water and a salt
Q30: What is the molarity of a KOH
Q31: What is the pH of a
Q33: The <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/.jpg" alt="The of
Q34: The neutralization reaction between <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/.jpg" alt="The
Q35: The conjugate base of <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/.jpg" alt="The
Q36: Which of the following is correctly identified?<br>A)
Q37: Identify the conjugate acid-base pairs in the