Multiple Choice
The 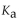 of
of 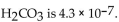
What is the pH of a buffer with 0.10 M 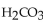 and 0.010 M
and 0.010 M 
A) 6.37
B) 7.37
C) 5.37
D) 7.63
E) 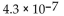
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q20: An acid and base react to form
Q21: Identify the <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/.jpg" alt="Identify the
Q22: What is the <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/.jpg" alt="What is
Q23: A patient with alkalosis has a blood
Q24: In a neutralization reaction, how many moles
Q26: Which of the following is a neutralization
Q27: Write the proper acid dissociation expression for
Q28: A solution which has <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/.jpg" alt="A
Q29: In a neutralization reaction,<br>A)water and a salt
Q30: What is the molarity of a KOH