Essay
Specify the hybridization of each carbon atom of limonene, a natural product present in citrus fruits, and thujone, which is derived from wormwood, a traditional component of the notorious liquor, Absinthe. 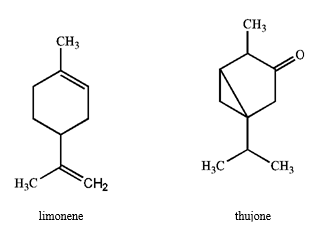
Correct Answer:

Verified
Correct Answer:
Verified
Q19: Covalent bonding<br>A) involves a transfer of electrons
Q29: Convert the skeletal drawing of the pharmaceutical
Q30: Instructions: Write valid Lewis (electron-dot) structures for
Q31: Instructions: Use the convention <span
Q32: Instructions: Refer to the following equation to
Q34: Instructions: Determine the hybridization for the indicated
Q35: Instructions: Determine the hybridization for the indicated
Q36: Consider the formation of an sp<sup>2</sup>
Q37: Instructions: Indole is pleasant smelling in highly
Q38: Instructions: Propose a structure for a molecule