Essay
Write an equation for the reaction of boron trifluoride, an important reagent in organic chemistry, with trimethylamine. Represent the movement of electrons with a curved arrow, and show the formal charges on the atoms in the product. 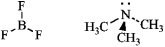
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: Instructions: Refer to the following equation to
Q3: Which of the following best represents the
Q5: Circle the Lewis bases in the group
Q6: How many nonbonding electron pairs are in
Q7: Instructions: Propose a structure for a molecule
Q8: Instructions: Consider the reaction below to answer
Q9: Instructions: Consider the two structures below
Q10: Which is the strongest base (pK<sub>a</sub> values
Q27: In drawing the Lewis structure for an
Q34: How many total valence electrons are represented