Multiple Choice
In the Lennard-Jones model of the hydrogen molecule, the potential is given by .In this model, the minimum internuclear separation, r0, is: 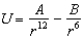
A) 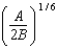
B) 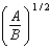
C) 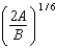
D) 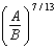
E) 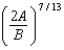
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q3: The energy gap for germanium is 0.670
Q22: The dissociation energy of the hydrogen molecule
Q23: A molecule makes a transition from the
Q24: The diagram below shows the distance between
Q25: The fundamental frequency of CO is 6.42
Q26: The rotation spectrum of the HCl molecule
Q27: The rotational kinetic energy of a
Q30: When a molecule jumps from a rotational
Q42: The energy released when an atom takes
Q45: The difference between donor and acceptor atoms