Multiple Choice
When a molecule jumps from a rotational energy level characterised by the rotational quantum number J to one characterised by J- 1, the difference in energy of levels J and J -1, EJ - EJ - 1, is:
A) 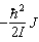
B) 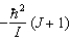
C) 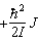
D) 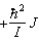
E) 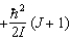
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q3: The energy gap for germanium is 0.670
Q22: The dissociation energy of the hydrogen molecule
Q25: The Fermi energy of a metal at
Q26: In comparing vibrational and rotational levels in
Q30: When a molecule jumps from a rotational
Q35: Because HF, hydrogen fluoride, is a covalent
Q37: The moment of inertia of a CO
Q38: To find the number of electrons per
Q40: The energy gap for a semiconductor is
Q45: The difference between donor and acceptor atoms