Multiple Choice
The wave functions of some molecules are a combination of wave functions with different values of the orbital quantum number .The wave function of PF5 combines s, p and d states in an sp3d hybrid orbital.We would expect such an overlap of wave functions in individual molecules to represent: 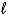
A) ionic bonding.
B) metallic bonding.
C) covalent bonding.
D) Van der Waal's bonding.
E) hydrogen bonding.
Correct Answer:

Verified
Correct Answer:
Verified
Q13: An energy band in a solid consists
Q14: A diatomic molecule consists of two point
Q15: What is the energy of the first
Q16: Assume the angular momentum of a diatomic
Q18: The fundamental frequency of HF is 8.72
Q20: An oxygen molecule has a moment of
Q21: The rotation spectrum of the HCl
Q22: In the hydrogen molecule, H2, the separation
Q23: A molecule makes a transition from the
Q24: The diagram below shows the distance between