Multiple Choice
When calculating the rotational kinetic energy of a diatomic molecule, with atoms of mass m1 and m2,the moment of inertia about an axis passing through the molecule's centre of mass, with r the atomic separation, is:
A) 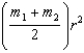
B) 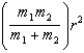
C) 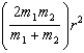
D) 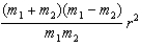
E) 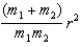
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: The smallest object one can distinguish using
Q5: The force constant of HCl is 480
Q6: A diatomic molecule consists of two point
Q7: A diatomic molecule consists of two point
Q8: If an electric field is applied to
Q10: Solid argon has a density of 1650
Q11: Assume a diatomic molecule can be considered
Q21: The energy of a molecule can normally
Q29: How many degrees of freedom does a
Q39: The Fermi temperature of copper is 80