Multiple Choice
The s, p, d, f, symbols represent values of the quantum number:
A) ms
B) n
C) 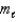
D) 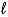
E) mj
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q4: When electrons fill a subshell in which
Q15: The Pauli Exclusion Principle states<br>A) no two
Q32: Quantum physics agrees with the classical physics
Q33: What is the difference in wavelength for
Q34: Which of the following, in which n
Q35: In 1921, Stern and Gerlach performed an
Q36: If P(r) is the radial probability density
Q38: How fast is the electron moving in
Q39: The ground state configuration of chlorine (Z
Q42: All quantum states forming a shell have