Multiple Choice
A gas expands as shown in the graph. If the heat taken in during this process is 1.02 * 106 J and 1 atm = 1.01 *105 N/m2, the change in internal energy of the gas (in J) is: 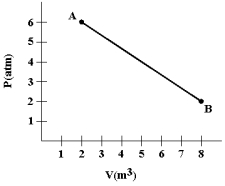
A) (-2.42 * 106)
B) (-1.40 * 106)
C) (-1.02 *06)
D) 1.02 * 106
E) 1.40 * 106
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q4: In an adiabatic free expansion<br>A) no heat
Q7: For an astronaut working outside a spaceship,
Q13: Which of the following statements is correct?<br>A)
Q24: The work done in the expansion from
Q25: Fred states that equal masses of
Q26: In an isothermal process:<br>A)P is constant.<br>B)V
Q27: A wall is constructed of a
Q27: Which statement below regarding the First Law
Q31: Gas in a container expands at a
Q33: A block of material of mass