Multiple Choice
A gas expands as shown in the graph. If the heat taken in during this process is 1.02 × 106 J and 1 atm = 1.01 × 105 N/m2, the change in internal energy of the gas (in J) is how much? 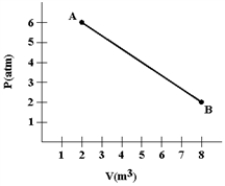
A) −2.42 × 106
B) −1.40 × 106
C) −1.02 × 106
D) 1.02 × 106
E) 1.40 × 106
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q6: A styrofoam container used as a picnic
Q7: The theorem of equipartition of energy states
Q8: A 300-g glass thermometer initially at 25°C
Q8: An 8000-kg aluminum flagpole 100-m high is
Q10: Which statement below regarding the first law
Q14: How much water at 20°C is needed
Q19: Duff states that equal masses of all
Q29: A 100-g cube of ice is heated
Q43: How much heat (in kcal) must be
Q49: If a person in Alaska were locked