Multiple Choice
Equal masses of hydrogen and helium gas are at the same temperature in vessels of equal volume. The atomic mass of helium is four times that of hydrogen. If the total mass of both gases is the same, the ratio of the pressure of helium (He) to that of hydrogen (H2) is
A) 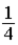 .
.
B) 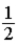 .
.
C) 1.
D) 2.
E) 4.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: Angela claims that she wears a cylindrical-shaped
Q13: One mole of an ideal gas has
Q21: A bicycle pump contains air at one
Q22: A gallon container is filled with gasoline.
Q25: A square plate has an area of
Q26: A helium-filled balloon has a volume of
Q27: Two thermometers are calibrated, one in degrees
Q29: A bowling ball size probe from a
Q33: Helium condenses into the liquid phase at
Q44: The pressure of a substance is directly