Multiple Choice
Solve the problem.
-The pressure of a gas varies jointly as the quantity of the gas (measured in moles) and the temperature and inversely as the volume of the gas. Suppose that the pressure of a particular gas is 1260 kiloPascals (kPa) when
The quantity is 5 moles , the temperature is 280 Kelvin (K) , and the volume is 600 cc. Find the pressure of that
Gas when the number of moles is 7, the temperature is 250 K, and the volume is 420 cc.
A) 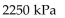
B) 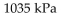
C) 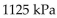
D) 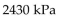
Correct Answer:

Verified
Correct Answer:
Verified
Q3: Simplify.<br>-<img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8475/.jpg" alt="Simplify. - A)
Q4: Simplify.<br>-<img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8475/.jpg" alt="Simplify. - A)
Q5: Solve the equation and check your solution.<br>-<img
Q6: Find the least common denominator for the
Q7: Answer true or false.<br>-<img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8475/.jpg" alt="Answer true
Q9: Solve the equation and check your solution.<br>-<img
Q10: Find the quantity indicated.<br>-f varies jointly as
Q11: Find the quantity indicated.<br>-z varies directly as
Q12: Simplify.<br>-<img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8475/.jpg" alt="Simplify. - A)
Q13: Solve the problem.<br>-While traveling at a constant