Multiple Choice
Solve the problem.
-The pH of a solution ranges from 0 to 14. An acid has a pH less than 7. Pure water is neutral and has a pH of 7. The pH of a solution is given by 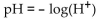 where
where 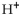 represents the concentration of the hydrogen ions in
represents the concentration of the hydrogen ions in
The solution in moles per liter. Find the pH if the hydrogen ion concentration is 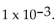
A) -3
B) 11
C) -11
D) 3
Correct Answer:

Verified
Correct Answer:
Verified
Q83: Evaluate.<br>-<img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8475/.jpg" alt="Evaluate. - A) 5
Q84: Find the unknown value.<br>-<img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8475/.jpg" alt="Find the
Q85: Use properties of logarithms to expand the
Q86: To what exponent must the base 10
Q87: Graph the function.<br>-<img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8475/.jpg" alt="Graph the function.
Q89: Use properties of logarithms to expand the
Q90: Evaluate.<br>-<img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8475/.jpg" alt="Evaluate. - A) 3
Q91: Find the power of 10. Round answers
Q92: Solve the equation without using a calculator.<br>-<img
Q93: Evaluate.<br>-<img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8475/.jpg" alt="Evaluate. - A)