Multiple Choice
Solve the problem.
-The pH of a solution ranges from 0 to 14. An acid has a pH less than 7. Pure water is neutral and has a pH of 7. The pH of a solution is given by 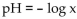 where x represents the concentration of the hydrogen ions in the
where x represents the concentration of the hydrogen ions in the
Solution in moles per liter. Find the hydrogen ion concentration if the pH = 12.
A) 
B) 0.08
C) 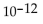
D) 2.48
Correct Answer:

Verified
Correct Answer:
Verified
Q2: A one-to-one function f is given. Find
Q3: Find the common logarithm of the number.
Q4: Evaluate.<br>-<img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8475/.jpg" alt="Evaluate. - A) 96
Q5: A one-to-one function f is given. Find
Q6: Find the unknown value.<br>-<img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8475/.jpg" alt="Find the
Q8: Solve the equation. Use a calculator where
Q9: For the given functions f and g
Q10: Use properties of logarithms to expand the
Q11: Solve the problem.<br>-The pH of a solution
Q12: Evaluate.<br>-<img src="https://d2lvgg3v3hfg70.cloudfront.net/TB8475/.jpg" alt="Evaluate. - A)