Short Answer
The following questions refer to the diagram below.The levels represent energy levels in a hydrogen atom.Each level is labeled with its energy (above the ground state of Level 1)in units of electron/volts (eV).The labeled transitions represent an electron moving between energy levels.
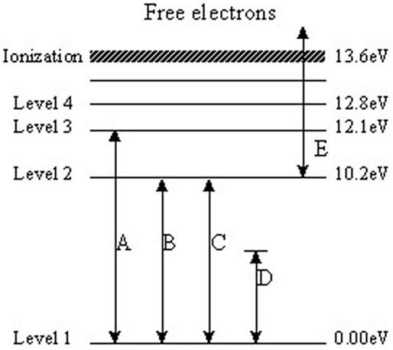
-Which transition represents an electron that absorbs a photon with 10.2 eV of energy?
Correct Answer:

Verified
Correct Answer:
Verified
Q33: Define atomic weight (or atomic mass).
Q34: Which of the following objects is not
Q35: Emission lines from different ionization states of
Q36: Process of Science: The theory of thermal
Q37: When light reflects off an object,what is
Q39: When an atom loses an electron,it becomes<br>A)vaporized.<br>B)dissociated.<br>C)ionized.<br>D)an
Q40: You observe the same spectral line in
Q41: Studying a spectrum from a star can
Q42: Laboratory measurements show hydrogen produces a spectral
Q43: We can see each other in the