Short Answer
The following questions refer to the diagram below.The levels represent energy levels in a hydrogen atom.Each level is labeled with its energy (above the ground state of Level 1)in units of electron/volts (eV).The labeled transitions represent an electron moving between energy levels.
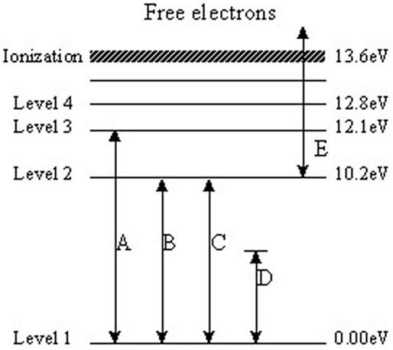
-Which transition represents the electron that emits a photon with the highest energy?
Correct Answer:

Verified
Correct Answer:
Verified
Q90: The most common isotope of oxygen has
Q91: Consider an atom of gold in which
Q92: The most common isotope of gold has
Q93: Suppose you built a scale-model atom in
Q94: Which forms of light are lower in
Q96: If you have a 100-watt light bulb,how
Q97: Briefly explain why spectral lines are useful
Q98: You observe a distant galaxy.You find that
Q99: Suppose you see two stars: a blue
Q100: The wavelength of a wave is<br>A)how strong