Short Answer
The following questions refer to the diagram below.The levels represent energy levels in a hydrogen atom.Each level is labeled with its energy (above the ground state of Level 1)in units of electron/volts (eV).The labeled transitions represent an electron moving between energy levels.
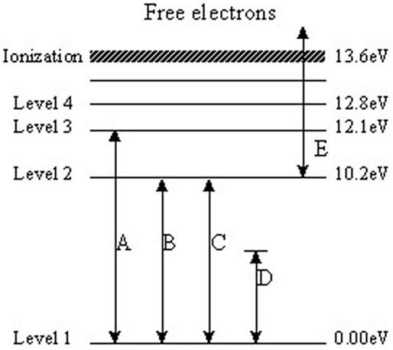
-Which transition represents an electron that is breaking free of the atom?
Correct Answer:

Verified
Correct Answer:
Verified
Q79: From shortest to longest wavelength,which of the
Q80: Which of the following statements about electrons
Q81: If you heat a gas so that
Q82: Suppose the surface temperature of the Sun
Q83: A perfectly opaque object that absorbs all
Q85: The study of energy levels in atoms
Q86: From laboratory measurements,we know that a particular
Q87: If two objects are the same size
Q88: Which of the following statements about X-rays
Q89: Suppose a photon has a frequency of