Multiple Choice
The reaction: 2 A( s ) + B( g ) 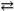 2 C( s ) represents a(n) :
2 C( s ) represents a(n) :
A) homogeneous equilibrium
B) heterogeneous equilibrium
C) instantaneous equilibrium
D) reaction that will not come to an equilibrium
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q22: What change in the reaction direction occurs
Q23: "Insoluble" salts are actually "sparingly" soluble with
Q24: Which of the following solids will provide
Q25: Potassium hydrogen phthalate (KHP) concentrations can be
Q26: Hydrogen is produced industrially by reacting propane
Q28: Which of following species is a Brønsted-Lowry
Q29: What is the pH of a 0.200
Q30: Unlike traditional concrete, engineered cementitious composites (ECC)
Q31: What is the molar solubility of AgCl
Q32: The production of ammonia N<sub>2</sub>( g )